Bone marrow transplants, which involve transplanting healthy blood stem cells, offer the best treatment for many types of cancers, blood disorders and immune diseases. Even though 22,000 of these procedures are performed each year in the U.S., much remains to be understood about how they work.
A new USC and Stanford study, conducted in mice, deepens the mystery, showing that successfully transplanted stem cells don’t behave “normally” as in a healthy person without a transplant. Instead, the radiation and high-dose chemotherapy used to wipe out diseased stem cells prior to transplantation appear to trigger “extreme behavior” in the newly transplanted cells.
The findings appeared in Proceedings of the National Academy of the Sciences (PNAS) on Jan. 8.
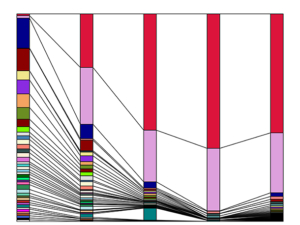
After radiation, a small number of blood stem cells make an outsized contribution to reconstituting the blood and immune system. (Figure by Jiya Eerdeng/Rong Lu Lab)
“Our research has important implications for understanding and optimizing bone marrow transplants and certain types of gene therapy,” said lead researcher and co-corresponding author Rong Lu, PhD, assistant professor of stem cell biology and regenerative medicine at the Keck School of Medicine of USC. The other co-corresponding author is Irving Weissman, MD, director of the Stanford Institute for Stem Cell Biology and Regenerative Medicine.
In a series of experiments, scientists learned that when transplanted into an irradiated mouse, only a very small minority of the stem cells produce blood and immune cells, while many other stem cells become dormant and do nothing. In addition, post-radiation, this handful of “super producer” stem cells also become biased toward producing only certain types of immune cells. However, the overall blood and immune system still tends to remain balanced.
In mice that had not undergone radiation, all stem cells contributed equally to the blood and immune systems, with the exception of T cells, suggesting that the preconditioning regimen used to ensure successful transplantation is the source of the abnormal cell behavior.
Additional co-authors include: Agnieszka Czechowicz and Jun Seita from Stanford; and Du Jiang from USC.
This research was supported by federal funding from the National Institutes of Health (grants R01-CA86065, U01-HL099999, K99/R00-HL113104, R01-HL135292, R01-HL138225, and P30-CA014089). Czechowicz received additional non-federal support from a Howard Hughes Medical Institute Medical Research Training Fellowship, a Stanford University Medical Scholars Fellowship and The Paul and Daisy Soros Fellowship for New Americans.
— Cristy Lytal


