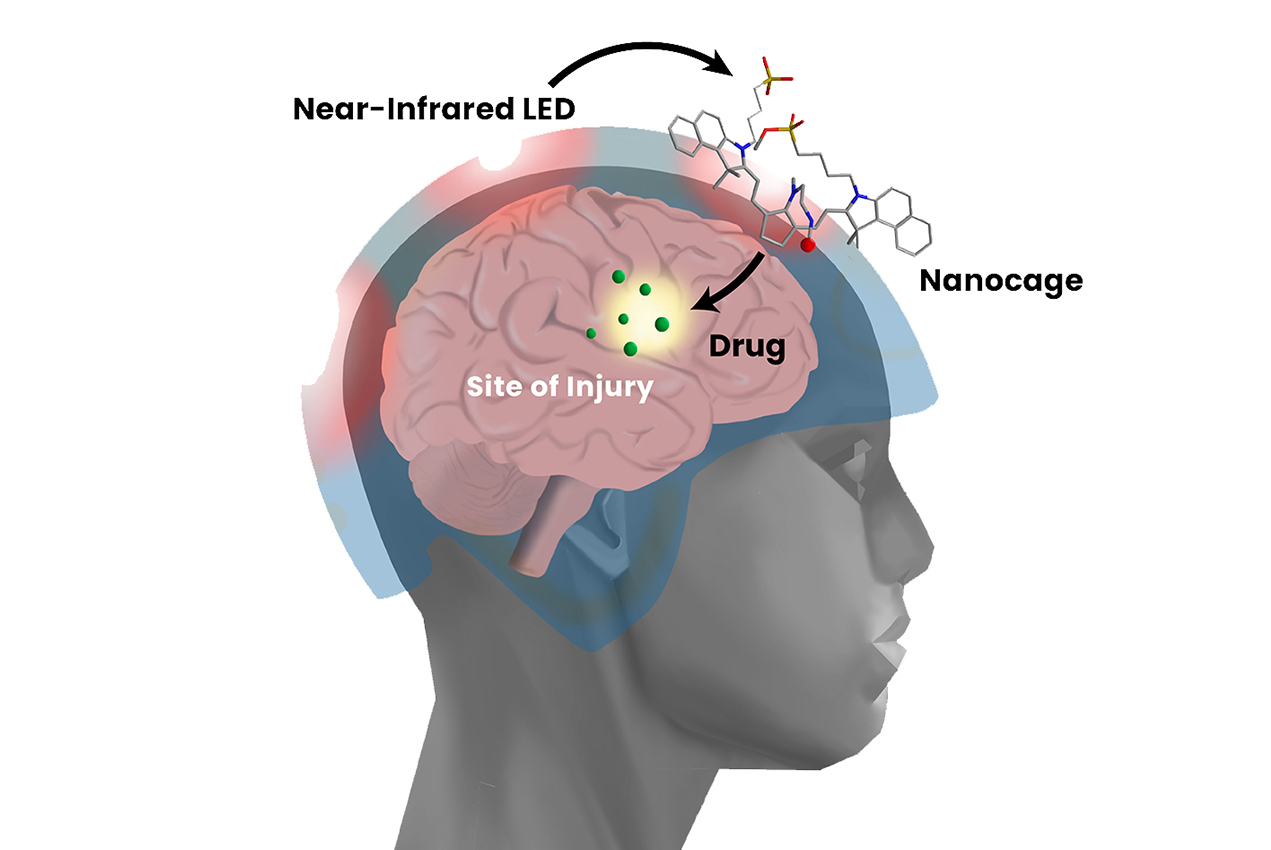
An interdisciplinary team of researchers at the University of Southern California has developed a precision drug delivery tool to selectively treat areas of the brain damaged during a traumatic brain injury (TBI). The researchers anticipate that their rapidly deployable intervention could potentially help prevent long-term brain damage in the millions of Americans who sustain a TBI each year.
This invention has been tested using two different therapeutics, and the results appear in the journals Frontiers in Chemistry and Molecular Pharmaceutics.
Stopping damage before it’s too late
In the United States alone, these injuries annually cause more deaths and lifelong disabilities than HIV/AIDS, breast cancer, multiple sclerosis and spinal cord injuries combined. Approximately one in three TBI patients die due to secondary complications. In 2014, an average of 155 Americans died following a TBI each day. The economic burden of treating these injuries and their long-term side effects is estimated to be $60-76.5 billion each year.
The causes of TBIs include falls, forceful sports collisions, car accidents and severe blows to the head, just to name a few. Side effects, such as cognitive challenges, dizziness, emotional changes and depression, can endure for days, weeks, or even years after the initial trauma. If effective treatments are not rapidly deployed, a TBI can trigger biochemical changes and inflammatory responses that progressively worsen a patient’s brain damage.
Despite the pressing need for effective and rapidly deployable therapies, there are currently no FDA-approved treatments specifically designed for TBIs. Some existing treatments rely on locating the injury within the skull and assessing the damage before proceeding, but this approach can be time consuming when acting quickly is key.
A nanoscale solution to a widespread problem
An ideal treatment approach for TBIs would involve a fast-acting, safe, transportable and easily administered drug that could permeate the brain’s protective barrier at the site of damage. The multidisciplinary team at USC working to solve this problem, led by researchers at the USC Dr. Allen and Charlotte Ginsburg Institute for Biomedical Therapeutics, has recently developed a novel drug delivery tool designed to safely and rapidly treat regions of the brain damaged during a TBI.
The tool itself is known as a nanocage, which confines therapeutic agents such as gabapentin and cyclosporine A and carries them within the body. Once a caged drug has migrated through a patient’s circulatory system and made its way to the brain, doctors can beam near-infrared (NIR) light through the patient’s skull to energize “open” the cage.
The researchers have found multiple benefits to this approach. Caging the drug before releasing it at the site of injury minimizes the side effects that one would expect from delivering an active drug throughout the entire body. Moreover, because the brain’s barrier is weakest at the site of the trauma, higher concentrations of the drug will enter the brain precisely at the site of damage —circumventing the need for time-consuming imaging to locate the damage before proceeding with first-line treatment.
The researchers designed the nanocage to be biodegradable and nontoxic. This emerging intervention is still in its preclinical stages, but the researchers anticipate that it can be packaged as a portable intervention for first responders to administer soon after the injury.
“I’m very hopeful for the impact this drug delivery tool could ultimately have on the millions of patients who experience TBIs each year,” said Mark Humayun, MD, PhD, University Professor of Ophthalmology and director of the USC Ginsburg Institute for Biomedical Therapeutics and the study’s principal investigator. “A rapid intervention like this could significantly improve patient outcomes, and our team is excited to continue testing this approach to hopefully bring it to the clinic soon.”
Caroline Black, PhD, lead author on the study, says this research was made all the more meaningful because of her personal connection to TBI within her family.
“My dad had a severe TBI when he was 17 and I’ve seen how the effects of secondary injury can persist decades after the initial trauma,” said Black, who is a USC-AbbVie postdoctoral fellow specializing in drug delivery sciences at the biopharmaceutical company AbbVie. “Our drug delivery tool has the potential to open new possibilities for rapid treatment of TBI, and I’m excited to see the impact it could have on improving patient outcomes.”
— Alexandra Demetriou
In addition to Humayun and Black, other researchers on the study include Caitlin M. DeAngelo, PhD, of the USC Department of Chemistry; Eugene Zhou, PhD, and Isaac Asante, PhD, of the USC School of Pharmacy; Stan G. Louie, PharmD, of the USC School of Pharmacy and the USC Dr. Allen and Charlotte Ginsburg Institute for Biomedical Therapeutics; and Nicos A. Petasis, PhD, of the USC Department of Chemistry, the USC School of Pharmacy and the USC Ginsburg Institute.