The USC Institute of Urology at Keck Medicine of USC is pioneering a new treatment option for men with intermediate risk prostate cancer that is minimally invasive, requires no radiation and entails minimal recovery time.
The procedure uses targeted high-intensity focused ultrasound, or HIFU to noninvasively target and kill cancer cells within the prostate, while the da Vinci robot removes the neighboring lymph nodes.
“HIFU has never been used in combination with robotic lymph node removal until now,” said Inderbir S. Gill, MD, Distinguished Professor and chair of urology, Shirley and Donald Skinner Chair in Urologic Cancer Surgery, founding executive director of the USC Institute of Urology and associate dean of clinical innovation at the Keck School of Medicine of USC. “This demonstration that we can use two completely different high-tech technologies to simultaneously address different aspects of the prostate cancer is a significant step forward.”
Until now, the HIFU procedure has been used mostly for men with low risk cancer because, although HIFU is effective at killing cancer cells inside the prostate, it cannot touch the lymph nodes. This dual procedure is performed under anesthesia, lasts about 3 – 3.5 hours and patients can potentially be discharged from the hospital the same day.
“This innovation offers the potential for ablative technologies to be used for more aggressive cancers”, said Andre Luis de Castro Abreu, MD, assistant professor of clinical urology and co-director, Center for Targeted Biopsies & Focal Therapy at the USC Institute of Urology. “Although this is only the initial experience, our novel concept adds another layer of confidence in the oncologic capability of HIFU.”
HIFU technology has been used in Europe for decades, but was only approved by the U.S. Food and Drug Administration in 2015. Gill noted that the USC Institute of Urology was the first, and is now the most experienced, academic medical center in the United States to utilize HIFU technology, and is the only center to offer HIFU treatments with both EDAP/Ablatherm and Sonacare/Sonablate devices.
“To date our patients have had minimal side effects, with quick recovery of potency and continence and return to normal activities,” said Daniel Park, PA, director of clinical operations at the USC Institute of Urology.
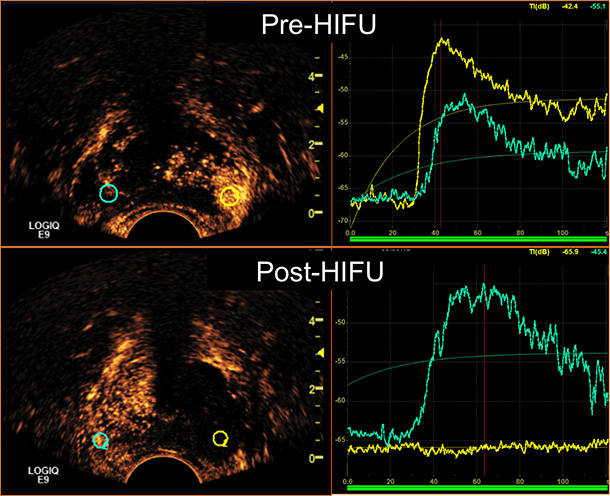
The team from the USC Institute of Urology also is developing an innovative contrast-enhanced ultrasound (CEUS), a microbubble-ultrasound technique to confirm whether the HIFU machine has ablated the targeted cancer area. Before the HIFU procedure, the patient is injected with a nontoxic type of microbubble that that is highly visible through real-time transrectal ultrasonography. Immediately after the HIFU procedure has been completed, a second microbubble injection is performed to assess the thoroughness of prostate ablation.
Gill added that the team at the USC Institute of Urology primarily performs this procedure on patients whose cancer is confined to one lobe of the prostate, thereby minimizing the risk of unwanted side effects including incontinence and impotence.
— Hope Hamashige