Gianluca Turcatel, PhD, and David Warburton, MD, of the Keck School of Medicine have joined fellow researchers at USC to develop a new tool to peer more deeply and clearly into living things, a visual advantage that saves time and helps advance medical cures.
It’s the sort of foundational science that can be used to develop better diagnostics and treatments, including detecting lung cancer or damage from pollutants. The technology is versatile enough to potentially be used for a smartphone app for use in remote medicine, food safety or counterfeit currency detection, said Francesco Cutrale, lead author of the study and research assistant professor of biomedical engineering at the USC Viterbi School of Engineering.
These scientists and more, affiliated with the USC Michelson Center for Convergent Bioscience, have been working on the technology for the past few years. Their findings have just been published in Nature Communications.
The technique focuses on the building blocks of biology. When biologists look deeply into a living thing, it’s not always clear what’s going on. Cells and proteins are deeply intertwined across tissues, leaving lots of questions about the interactions between components. The first step to curing disease is seeing the problem clearly, and that’s not always easy.
How the new imaging technology works
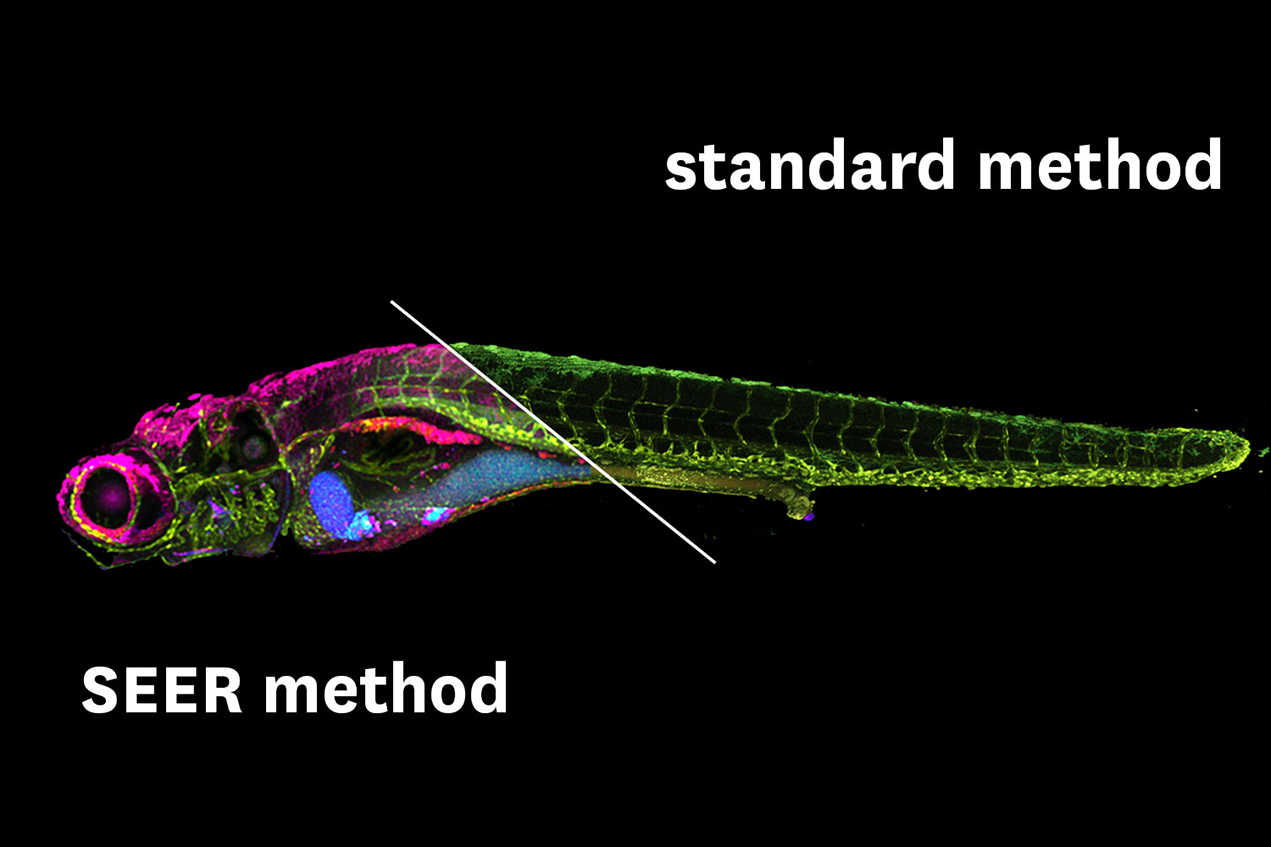
Researchers have been relying on a technique called fluorescence hyperspectral imaging (fHSI). It’s a method that can differentiate colors across a spectrum, tag molecules so they can be followed, and produce vividly colored images of an organism’s insides.
But the advantages that fHSI offers come with limitations. It doesn’t necessarily reveal the full-color spectrum. It requires lots of data, due to the complexity of biological systems, so it takes a long time to gather and process the images. Many time-consuming calculations are also involved, which is a big drawback because experiments work better when they can be done in real time.
To solve those problems, the USC researchers developed a new method called spectrally encoded enhanced representations (SEER). It provides greater clarity and works up to 67 times faster and at 2.7 times greater definition than present techniques.
It relies on mathematical computations to parse the data faster. It can process vibrant fluorescent tags across the full spectrum of colors for more detail. And it uses much less computer memory storage, even more important with the explosion of big data research behind modern convergent bioscience research. According to the study, SEER is a “fast, intuitive and mathematical way” to interpret images as they are collected and processed.
From detecting lung cancer to a potential smartphone app
SEER’s first application will be in the medical and research field. The versatile algorithm, first authored by Wen Shi and Daniel Koo at the Translational Imaging Center of USC, will be used for detecting early stages of lung disease and potential damage from pollutants in patients in a collaboration with doctors at Children’s Hospital Los Angeles.
Improvements in imaging technologies can also reach the consumer level, so it’s likely that technologies such as fHSI and SEER could be installed on smartphones to provide powerful visualization tools.
The Michelson Center brought together the diverse research team responsible for this development thanks to a generous $50 million gift from orthopedic spinal surgeon, inventor and philanthropist Gary K. Michelson and his wife, Alya Michelson.
— Gary Polakovic
The study authors include Cutrale and Wen Shi, Daniel Koo, Masahiro Kitano, Hsiao Chiang, Le Trinh, Cosimo Arnesano and Scott Fraser of USC; Gianluca Turcatel and David Warburton of the Keck School and the Saban Research Institute of Children’s Hospital Los Angeles; and Benjamin Steventon of the University of Cambridge.
The study was supported by a Department of Defense grant (PR150666) and USC.