Studying zebrafish, investigators at The Saban Research Institute and The Heart Institute of Children’s Hospital Los Angeles have discovered a new source for cells that can develop into coronary vessels and have identified the signaling protein, a chemokine called CXCL12, which guides this process.
Results of the study were published online May 26 by the journal Developmental Cell.
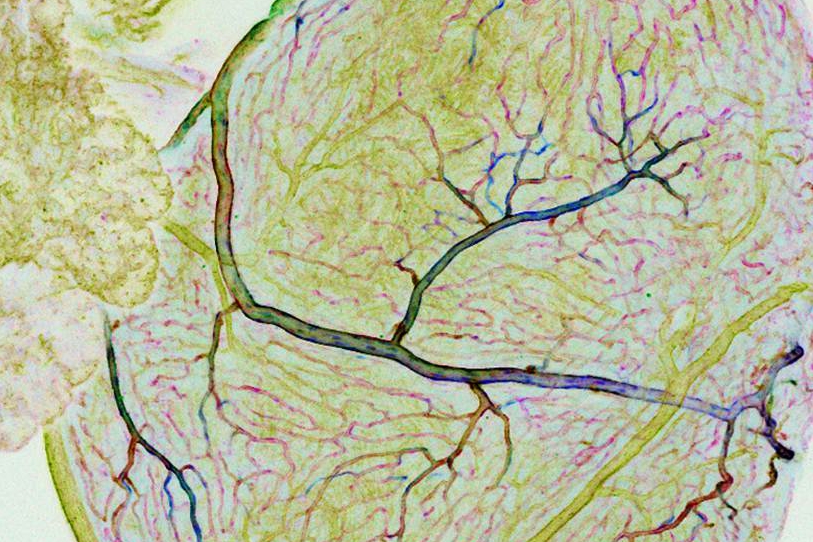
The heart has its own dedicated blood supply, with coronary arteries that supply oxygen-rich blood to the heart and cardiac veins that remove the deoxygenated blood. This system of vessels nourishes the heart, enabling it to pump blood to all the other organs and tissues of the body. Yet despite their critical importance, the process and molecules required for coronary vessel development have not been fully determined.
Zebrafish have emerged as an important vertebrate model for cardiovascular research for a number of reasons, including the ability to regenerate its heart if damaged, and because the transparency of the embryos allows easy observation of internal processes like blood vessel development.
Using confocal and time-lapse imaging, the investigators were able to visualize coronary vessels developing from the endocardium, or the inner lining of the heart – specifically from the atrioventricular canal, the structure that divides the heart into compartments.
“This furthers our efforts into heart regeneration to repair human hearts,” said Ching-Ling (Ellen) Lien, PhD, principal investigator at the Saban Research Institute of Keck Medicine of USC-affiliated Children’s Hospital Los Angeles and senior author on the paper. “We have now found a novel source of cells that can differentiate into coronary vessels and have identified the factors required.”
Lien and her team observed that zebrafish with a mutation at the CXCR4 receptor survive but are not able to form coronary vessels or undergo heart regeneration following injury. Since fish without this mutation are able to do both, the investigators concluded that an interaction between CXCR4 receptors on endothelial cells and the CXCL12b protein expressed by the myocardium regulate the process.
In addition to providing basic information about the developing heart, this finding may also have clinical relevance.
“Children or young adults may not be aware of having abnormal coronary vessels because their circulation is adequate until the heart is stressed by increased demands, for instance when participating in strenuous sports,” explains Lien, “Then suddenly, an apparently healthy, young person dies. Alternatively, a person with abnormal coronary vessels might have higher risk of experiencing heart attacks later on in life. Our findings will guide future study toward understanding these devastating conditions in order to be better able to diagnose them and develop interventional strategies.”
The first author, Michael R.M. Harrison, is a CIRM scholar and Saban RCDF fellow. Additional contributors include Ying Huang and Arthela Osorio of the Saban Research Institute; Jeroen Bussmann and Arndt F. Siekmann, Max Planck Institute for Molecular Biomedicine, Muenster, Germany; Long Zhao, C. Geoffrey Burns and Caroline E. Burns, Harvard Medical School; and Henry M. Sucov, Broad Center for Regenerative Medicine and Stem Cell Research at USC.
The study was supported in part by the National Heart, Lung and Blood Institute, the Saban Research Institute Career Development Award and a California Institute for Regenerative Medicine (CIRM) postdoctoral fellowship.
— Ellin Kavanagh, CHLA